New Fibroid Treatments – Oriahnn and Myfembree
On May 29, 2020, the FDA approved ORIAHNN, the first ever nonsurgical treatment specifically indicated for heavy menstrual bleeding due to fibroids in premenopausal women. More recently, a second medication, Myfembree was released.
Uterine fibroids are the most common benign tumors in women of reproductive age, with heavy menstrual bleeding being one of the most important symptoms prompting women to seek treatment. Fibroids are the leading cause of hysterectomy in the US and, although surgical treatments are available, many women may prefer not to have surgery. Now, ORIAHNN and Myfembree may be a medical treatment option for these women.
ORIAHNN and Myfembree are FDA-approved oral medications specifically made to help lighten heavy periods due to uterine fibroids
These medications are different. It’s not a surgery, procedure, or birth control. It’s an oral medication that’s clinically proven to reduce heavy menstrual bleeding due to uterine fibroids in as soon as 1 month.
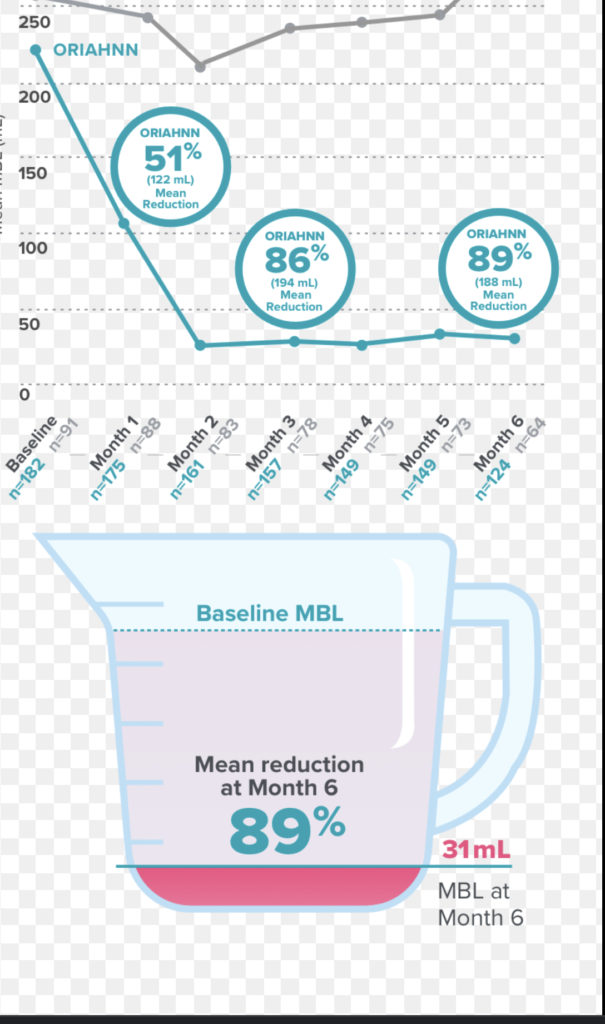
ORIAHNN clinical trial results
ORIAHNN was studied in two 6-month clinical trials, including premenopausal women with heavy periods due to uterine fibroids aged 25-53.
ORIAHNN was proven to lighten heavy periods in about 7 in 10 women
This meant bleeding volume was reduced by at least 50% from start of trial, and to below 80 mL (about 1/3 of a cup) at the final month of treatment. *Compared with about 1 in 10 women taking placebo.
Average bleeding was reduced by about 89% (-188 mL). * Compared with an increase of about 9% (+12 mL) in women taking placebo. Results shown are from start of trial to Month 6. These results are from Trial 2. Trial 1 results were similar.
Average periods were reduced quickly—by half at Month 1 and about 85% at Month 3