It is important to remember that not all women with fibroids who have heavy bleeding are bleeding because of the fibroids. A small intramural fibroid or large pedunculated fibroid (one that is hanging off the uterus) are not likely causing heavy bleeding. One option for managing heavy bleeding in women with or without fibroids is the Mirena intrauterine system. This progesterone releasing IUD is a highly effective method of birth control and is the most commonly used reversible method worldwide. It is placed in the uterus during an office visit and immediately begins releasing a low and steady dose of progesterone into the lining of the uterus. The IUD acts in a number of ways to prevent pregnancy. It also thins the lining of the uterus, an effect that eventually leads to lighter periods. In fact, after a few months, many women with stop menstruating.
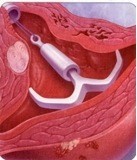
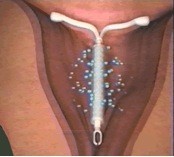
The FDA recently approved the Mirena IUD for menorrhagia or heavy menstrual bleeding. FDA approval is based on data from a randomized, open-label, active-control, parallel-group clinical trial of 160 healthy women of reproductive age who had confirmed heavy menstrual bleeding and did not have any medical conditions known to cause HMB, with the exception of small uterine fibroids in some patients. Heavy menstrual bleeding, defined as menstrual blood loss (MBL) of ≥ 80 mL, was determined using the alkaline hematin method. In the study, Mirena (n=79) was compared to an approved first-line hormonal therapy, medroxyprogesterone acetate (n=81), over six menstrual cycles. Successful treatment was defined when two outcomes were met: 1) a proportion of subjects with end-of-study MBL < 80 mL and 2) a ≥ 50% decrease in MBL from baseline to end-of-study. Mirena demonstrated a significantly superior reduction in MBL. Additionally, a greater number of women in the Mirena arm achieved successful treatment vs. those in the medroxyprogesterone acetate arm (85% vs. 22%: respectively, p=<0.001). The study excluded women with organic or systemic conditions that may cause heavy uterine bleeding (except fibroids, with total volume not > 5 mL). The most common reported adverse events for Mirena in the study were uterine bleeding/spotting at irregular intervals, headache, ovarian cysts, vaginitis, pain during menstruation (dysmenorrhea), pelvic pain, and breast tenderness.
